Research in the Kloosterman Lab focuses on understanding spatial learning and memory processes in the brain. Our starting point is often the hippocampus — a brain region famous for its role in long-term memory and the discovery of place cells. In our recent studies, we look at the role of reward in learning, the interactions between the hippocampus and (sub)cortical areas, and the contribution of memory replay to cognition.
Reward magnitude modulates memory consolidation and plasticity of spatial representations in the hippocampus
Publications
Single-trial dynamics of hippocampal spatial representations are modulated by reward value Journal Article
In: Current Biology, vol. 31, no. 20, pp. 4423–4435, 2021.
A Dual Reward-Place Association Task to Study the Preferential Retention of Relevant Memories in Rats Journal Article
In: Frontiers in Behavioral Neuroscience, vol. 14, pp. 69, 2020.
Post-learning Hippocampal Replay Selectively Reinforces Spatial Memory for Highly Rewarded Locations Journal Article
In: Current Biology, vol. 29, no. 9, pp. 1436–1444, 2019.

In reinforcement-based spatial learning, subjects learn across multiple experiences that some actions are more likely to result in success (reward) than other actions. In general, larger rewards lead to stronger and longer-lasting memories, likely because reward value modulates the learning process. In a series of studies, we investigated the neural mechanisms that support the effect of reward size on spatial learning. We developed a new task in which rats learn multiple place-reward associations, enabling a direct comparison between high-value and low-value experiences (Michon et al. (2020)). During learning of the reward location, the brain’s representation of space (as manifested in hippocampal place cell activity) is continuously updated and high reward value accelerates the updating process (Michon et al. (2021)). Next, we showed that the differential processing of high and low reward value experiences extends to sleep periods (Michon et al. (2019)). Neural ensemble recordings revealed that sleep reactivation of hippocampal ensembles that code for the path to reward occurred more frequently for high reward value. Temporally specific disruption of reactivation events using our custom real-time data analysis platform erased the benefit that high reward value has on memory retention. These findings indicate that sleep reactivation serves to protect the high-value memory traces from degradation or interference.
Sharp-wave ripple associated activity in the medial prefrontal cortex supports spatial rule switching
Publications
Sharp-wave ripple associated activity in the medial prefrontal cortex supports spatial rule switching Unpublished
bioRxiv 2022.11.03.515023, 2022.
Hippocampal memory replay engages a broad network of brain regions, but it is unknown how the brain’s reactivation process is coordinated across cortical and subcortical circuits and whether the such coordination is instrumental in spatial learning and memory consolidation. In a recent study, we looked at the interaction between the hippocampus and the prefrontal cortex in the context of behavioral flexibility — that is, the ability to adjust one’s actions if the circumstances change. We showed that prefrontal activity immediately following hippocampal putative replay events is required for animals to successfully adapt to a rule change in a spatial memory task (Den Bakker et al. (2022)).
Awake replay is chunked, associated with reward signals, but may not be necessary for short-term memory
Publications
Awake hippocampal replay is not required for short-term memory Unpublished
bioRxiv 2022.11.03.514989, 2022.
VTA neurons coordinate with the hippocampal reactivation of spatial experience Journal Article
In: Elife, vol. 4, pp. e05360, 2015.
Hippocampal replay of extended experience Journal Article
In: Neuron, vol. 63, no. 4, pp. 497–507, 2009.
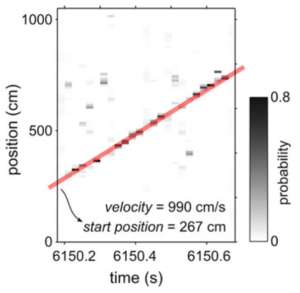
In an early study, we showed that place cells that form the hippocampal spatial map (see short article) are reactivated whenever animals briefly pause during running on a track (for example to eat reward). We showed this using a neural decoding approach that translates the cell activity into an estimate of the animal’s location (Davidson et al. (2009); Kloosterman (2011)). There are various hypotheses that attempt to explain the role of this hippocampal replay in memory. Our initial analysis showed that memory traces of long-duration experiences are reactivated at high and constant speed in short, concatenated chunks (Davidson et al. (2009)). This pattern may allow for the flexible expression of memory traces that guide future behavior. In a follow-up study, we revealed that the brain’s reward system is coordinated with hippocampal replay, indicating that a wider network of brain regions participates in the reactivation process, which may contribute to the reinforcement of recent rewarded experiences (Gomperts et al. (2015)). Very recently, we used our real-time platform Falcon to specifically disrupt activity at the time of all putative awake replay events (Deceuninck and Kloosterman (2022)) to demonstrate that hippocampal replay is not needed for short-term spatial memory, contrary to what was hypothesized before.