In the Kloosterman lab, we develop new technologies and experimental approaches to reveal the neural basis of brain function and behavior. Our contributions include novel types of neural probes and brain implants for long-term measurement of cellular activity at large scale, the development of neural decoding approaches and other data analysis techniques to reveal the brain’s computations and software for real-time analysis of brain signals that can be used in brain-computer interfaces.
Neural probes and brain implants for large-scale recording of cellular activity in behaving rodents
Publications
Implantation of Neuropixels probes for chronic recording of neuronal activity in freely behaving mice and rats Journal Article
In: Nature Protocols, vol. 16, no. 7, pp. 3322–3347, 2021.
System for recording from multiple flexible polyimide neural probes in freely behaving animals Journal Article
In: Journal of Neural Engineering, vol. 17, no. 1, pp. 016046, 2020.
Integration of silicon-based neural probes and micro-drive arrays for chronic recording of large populations of neurons in behaving animals Journal Article
In: Journal of Neural Engineering, vol. 13, no. 4, pp. 046018, 2016.
Micro-drive array for chronic in vivo recording: drive fabrication Journal Article
In: JoVE, no. 26, 2009.
3D design files
tetrode drive implant (2009) — Solidworks design files and 3D print files
neuropixels implant (2021) — 3D print files

We have designed 3D-printed implants that facilitate neuronal recordings in freely behaving mice and rats, including implants designed for a new generation of high-density Neuropixels probes (Van Daal et al. (2021)). These custom implants have enabled us to implant probes at multiple defined locations in the brain, to perform chronic recordings during long term learning tasks, and to conveniently reuse the probes.
To improve long-term stability of recordings with neural probes, we develop flexible polyimide-based probes that inflict less damage on the brain. The main challenge that we are tackling is the ability to manufacture flexible probes with a large number of recording electrodes. This work is performed in collaboration with the research division Micro- and Nanosystems at KU Leuven and ATLAS Neuroengineering.
Algorithms for neural decoding of real and imagined position
Publications
Kernel density compression for real-time Bayesian encoding/decoding of unsorted hippocampal spikes Journal Article
In: Knowledge-Based Systems, vol. 94, pp. 1–12, 2016.
Bayesian decoding using unsorted spikes in the rat hippocampus Journal Article
In: Journal of Neurophysiology, vol. 111, no. 1, pp. 217–227, 2014.
Analysis of hippocampal memory replay using neural population decoding Book Chapter
In: Fellin, T.; Halassa, M. (Ed.): Neuronal Network Analysis, vol. 67, Humana Press, 2011.
Hippocampal replay of extended experience Journal Article
In: Neuron, vol. 63, no. 4, pp. 497–507, 2009.
Software
no-spike-sort compressed decoding — source code and documentation
no-spike-sort decoder on GPU (Hu et al. 2018) — source code
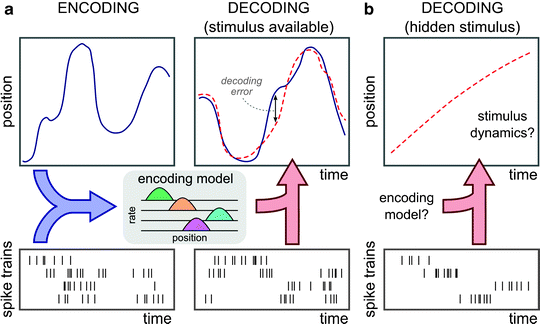
Computations in the brain are supported by the communication between neurons through electrical impulses (spikes). To facilitate the study of experience-dependent reactivation in neuronal ensembles, we developed novel neural decoding approaches that make efficient use of available information in the raw spiking data. We first showed that decoding can be used successfully to reveal “hidden” representations in the brain, such as hippocampal replay of place cell sequences (Davidson et al. (2009); Kloosterman (2011)). Most spike-based decoding approaches first require a spike-sorting step that separates the contribution of individual neurons. However, this step is time consuming and generally leads to loss of information. We developed a new decoding algorithm that works directly on the raw spikes without the spike-sorting step, resulting in improved decoding performance (Kloosterman et al. (2014)). In a follow-up study, we significantly sped up the computation for spike-sorting-less decoding (Sodkomkham et al. (2016)), such that the algorithm can be used in real-time on streaming data (see Ciliberti et al. (2018)).
Software platform “Falcon” for closed-loop neuroscience
Publications
Real-time classification of experience-related ensemble spiking patterns for closed-loop applications Journal Article
In: Elife, vol. 7, 2018.
Falcon: a highly flexible open-source software for closed-loop neuroscience Journal Article
In: Journal of Neural Engineering, vol. 14, no. 4, pp. 045004, 2017.
Software
Falcon — source code and documentation
Falcon extensions — source code and documentation
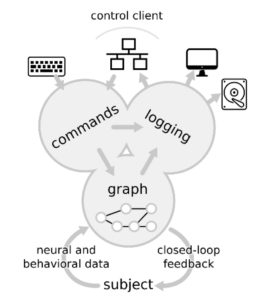
We built a modular software platform (Falcon) for real-time neural signal analysis that enables the detection and perturbation of neural events at millisecond timescales. The software executes user-defined graphs of connected data processing blocks and has a modular and extendable design.
Falcon can be used to reveal the causal role of specific neural activity patterns in cognitive processes and behavior. For example, we have used Falcon to perturb bursts of fast oscillations (sharp-wave ripples) in the hippocampus and demonstrate their contribution to memory consolidation (Michon et al. (2019)) and rule learning (Den Bakker et al. (2022)), but not tasks that only require short-term memory (Deceuninck and Kloosterman (2022)).
We have extended Falcon with a online implementation of our no-spike-sort decoding algorithm. With this extension, we experimentally demonstrated the real-time detection and classification of hippocampal memory replay patterns (Ciliberti et al. (2018)).